The diagram below describes the steps involved in gaining approval to access NSW data.
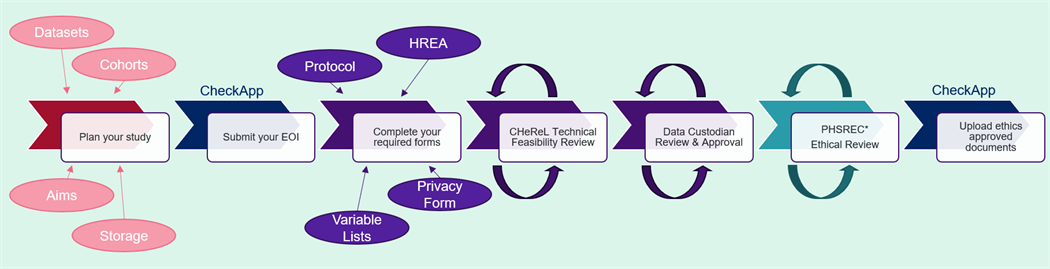
The application process is iterative. Timeframes for Technical Feasibility Review, Data Custodian Review and Approval, and Ethical Review and Approval vary significantly depending on the complexity of your application and the number of feedback and review cycles required.
More detailed information about each step can be viewed via the ‘Read more’ button. For pricing information please . If you are seeking to access unlinked data from any of our MLK datasets, please contact the .
The steps below detail the process for all research related linked data requests.
Step
01
of 7
Dataset and variable information can be found on the CHeReL website.
NSW Health recommends disclosing unit record health data intended for secondary use by external parties into a suitable Secure Data Environment (SDE). Consequently, all relevant new data projects are required to comply with this recommendation.
For additional details regarding suitable Secure Data Environments (SDE) and when they are recommended, please refer to this website: https://www.health.nsw.gov.au/data/sharing/Pages/secure-access-environments.aspx
Step
02
of 7
Submit an Expression of Interest via CheckApp and complete your application forms
Step
03
of 7
When your submission is ready, please email the completed forms to the Research Project Managers
Please email your documentation to MOH-cherel@health.nsw.gov.au.
We will review your application and provide feedback within 2-4 weeks.
Please note, for some non-NSW Health data requests you will need to contact the data custodians/organisation directly to confirm whether additional application requirements apply.
Step
04
of 7
Once your team has addressed any feedback from the Research Project Manager, your project will be deemed technically feasible.
You will receive an email to confirm technical feasibility and you can upload this to REGIS as part of your submission to PHSREC.
Step
05
of 7
The team at CHeReL will seek data custodian approval on your behalf for data sets in the Master Linkage Key.
The CHeReL team will seek data custodian approval on your behalf for datasets included in the Master Linkage Key.
- Allow at least 2–4 weeks for review and feedback from Master Linkage Key data custodians.
- CHeReL will forward data custodian responses to you as they are received.
- Be prepared to respond to feedback from data custodians. Changes to your project may be required.
- For all other data collections, you are responsible for obtaining data custodian approvals. Please use consistent documentation across all submissions. Contact the CHeReL team if you need assistance.
Step
06
of 7
Submit your data custodian approvals, technical feasibility letter and all application documents to PHSREC.
Please submit all documents to PHSREC via REGIS. PHSREC and other HRECs may require additional documentation. Please consult the relevant HREC and allow sufficient time to prepare other forms.
Step
07
of 7
Once you receive your Ethics approval, please send us the approval letter and a copy of all documents listed on the approval letter.
The diagram below describes the steps involved in gaining approval to access ACT data, more detailed information about each step can be viewed via the ‘Read more’ button.
Steps 1 to 3 are the same in NSW and ACT
Step
01
of 7
Dataset and variable information can be found on the CHeReL website.
Step
02
of 7
Here are the forms you need to complete if you are applying for NSW and ACT linked data:
Step
03
of 7
When your submission is ready, please email the completed forms to the Research Project Managers at: moh-cherel@health.nsw.gov.au.
We will review your application, provide feedback and a quote. Please allow 2-4 weeks for us to respond.
Please note, the NSW Central Cancer Registry (NSW CCR) and the 45 and Up Study may charge a fee for data extraction which is not included in the CHeReL quote.
For more information on cost of requesting CCR, please contact the Cancer Institute NSW at:
CINSW-DARenquiries@health.nsw.gov.au
For more information on cost of requesting data from the 45 and Up Study, please contact the Sax Institute at: 45andup.research@saxinstitute.org.au.
Step
04
of 7
Once your team has addressed any feedback from the Research Project Manager, your project will be deemed technically feasible.
You will receive an email to confirm technical feasibility. ACT Health will ask for this during their approval process.
Step
05
of 7
Submit your data custodian approvals, technical feasibility letters, application documents and other supporting documents to the appropriate ethics committee.
If you have NSW data in your project, you should submit to PHSREC first.
If your project has ACT-only data, you should submit to ACT Health HREC first.
Please check each ethics committee website for their requirements.
Step
06
of 7
Obtain data custodian sign off for ACT data sets.
Once you receive notice of ethics approval from the ACT Health HREC, please contact relevant data custodians to obtain sign off. ACT Health will advise on this process.
Step
07
of 7
When you obtain both ethics and data custodian approvals, please notify the CheReL and send us a copy of all documents listed on your approval letters. Please send this to moh-cherel@health.nsw.gov.au
 New South Wales Health
New South Wales Health



